Direct-to-Docs Opioid Marketing
Pharmaceutical industries are marketing opioids directly to physicians. How much of it contributes to current opioid addiction epidemic?
Read Time: 2 minutes
Published:
One factor that has fueled the current addiction epidemic is the pharmaceutical industry’s direct-to-physician marketing of opioids. To promote new drugs, pharmaceutical companies often market their products to physicians by providing industry-sponsored meals, grants, and subsidies for continued medical education and training. Physicians have also received fees for consulting or speaking publicly about opioid products, which could increase prescriptions among their peers.

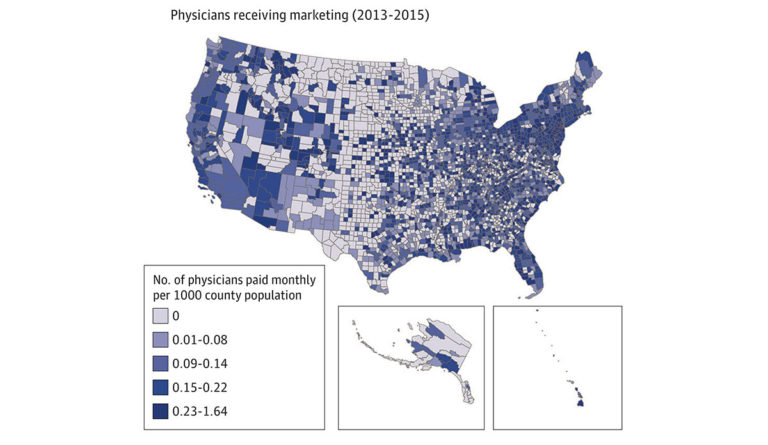
A recent study sought to understand the relationship between mortality from opioid overdose and the pharmaceutical industry’s direct marketing of opioids to physicians. The study analyzed the association between three factors in every US county: the amount of marketing payments pharmaceutical companies made to physicians, opioid prescribing rates, and the number of overdose deaths.
Photo via Scott E. Hadland, Ariadne Rivera-Aguirre, Brandon D. L. Marshall, and et al., Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. JAMA Network.
Researchers found that direct marketing of opioids to physicians was associated with increased opioid prescribing rates and increased overdose mortality one year after marketing engagements. Figure 1 illustrates the number of physicians who received direct marketing between 2013 and 2015. Figure 2 highlights overdose mortality rate across the US one year later (2014-2016). Higher prescribing rates facilitated the link between opioid marketing to physicians and mortality from overdoses.
The national response to the opioid epidemic has focused in part on reducing the number of opioids prescribed by physicians. Additionally, the Physician Payments Sunshine Act promotes financial transparency between pharmaceutical companies and healthcare providers. The Act requires any payments between both entities to be reported to the Centers for Medicaid and Medicare Services. States that have additional reporting requirements or limitations beyond those stipulated by the Physician Payments Sunshine Act include Vermont, Massachusetts, Minnesota, Washington DC, West Virginia, California, Connecticut, Louisiana, and Nevada.
By increasing regulation around pharmaceutical direct-to-physician marketing and making pharmaceutical company payments to physician available to the public, states have the potential to reduce overdose mortality.
Databyte via Scott E. Hadland, Ariadne Rivera-Aguirre, Brandon D. L. Marshall, and et al., Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. JAMA Network.



