Antibiotic Resistance Is Catching Up
By 2050, resistant infections could kill more people worldwide than cancer. But this outcome is preventable if we act now.
Read Time: 4 minutes
Published:
A sore throat, a sinus infection, or a scraped knee used to be routine care with predictable treatments. Today, antimicrobial resistance (AMR) makes common infections harder to treat. In the United States, antibiotic-resistant bacteria cause more than 2.8 million infections and are responsible for over 35,000 deaths each year. Worldwide, bacterial resistance was directly responsible for over 1.27 million deaths in 2019 and associated with 4.95 million deaths. If we do not scale up prevention measures, new projections estimate about 1.9 million deaths directly attributable to AMR and 8.2 million associated deaths in 2050.
Antibiotic resistance is growing for a number of reasons. Sometimes, antibiotics are prescribed for viral illnesses, such as colds or flu, even when they offer no benefit. Access to antibiotics is also easier than ever, as many antibiotics are purchasable even without a prescription. Patients with bacterial infections sometimes stop treatment early once they start to feel better, which leaves the toughest microbes behind to multiply. In clinics and urgent care centers, time pressure and patient expectation can nudge prescribers toward “just in case” antibiotics.
Animal husbandry, the practice of breeding and raising livestock for food production, is also contributing to the rise of AMR. Many farmers who raise livestock give antibiotics to prevent disease in crowded settings or to speed growth, exposing entire herds and flocks when animals are not sick. Resistant bacteria then spread through slaughter and processing, manure runoff, and worker contact.
Nearly two-thirds of projected AMR deaths in 2050 are expected among those 70 and older.
Christopher Murray and colleagues analyzed 24 bacterial pathogens and 84 pathogen-drug pairs, drawing on 20 million data points from 204 countries and territories. Each pair links a bacterium to a standard antibiotic. For every pair, they estimated how often the bacteria were not susceptible to that drug, then applied those rates to infection counts. When viewed together, the 84 pairs provide a consistent picture of resistance across pathogens and settings. This type of analysis can quantify deaths attributable to resistance (cases in which patients likely would have survived had the bacteria responded to the antibiotic) and deaths associated with the disease (cases in which resistance worsened outcomes by delaying or limiting effective treatment).
Researchers projected South Asia and Latin America to face the highest rate of all-age mortality rates attributable to AMR by 2050. The key reasons for this are a dangerous combination of an aging population, limited health care infrastructure, and widespread antibiotic misuse, which create ideal conditions for resistant bacteria to thrive. Age raises risk because older adults have more contact with health care and are more likely to have chronic conditions that require treatment, which increases exposure to antibiotics and to health care-associated pathogens. Nearly two-thirds of projected AMR deaths in 2050 are expected among those 70 and older.
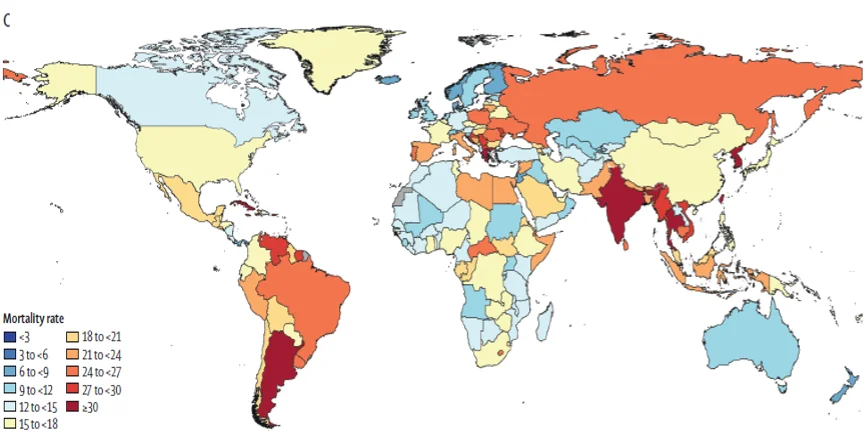
AMR is a problem regardless of a country’s income level, with high-income countries also seeing rising resistance in community and hospital settings, as shown in the map above. Methicillin-resistant Staphylococcus aureus, or MRSA, can move through households, locker rooms, and schools. A small cut or scrape can become a serious infection when resistant staph gains a foothold.
The trajectory of antibiotic resistance is still ours to change if we act now.
Public health tools to monitor and evaluate AMR already exist. The WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) is a shared system among 109 countries and territories that helps track how often antibiotics are used and how well they work. Local lab summaries, often called antibiograms, show which antibiotics beat the germs most common in that community. Clinicians use these summaries to pick the best drug response.
Analyzing microbial DNA also offers a unique place of intervention. Genomic sequencing tells us which resistant bacteria are present and how they spread from place to place. When labs share these results through national systems and GLASS, health officials can see where resistant organisms are gaining ground and prevent outbreaks from occurring in the first place.
Progress depends on scaling best practices, not ticking off a checklist. High-quality care means quick, accurate diagnosis, choosing the right antibiotic, and finishing the full course prescribed. Prudent use means treating confirmed or strongly suspected bacterial illness, not viral infections. Modeling suggests that scaling these practices through 2050 could avert about 92 million deaths. Developing new antibiotics for the toughest infections could prevent another 11 million. These figures reflect potential gains, not guaranteed results.
By 2050, resistant infections could kill more people than cancer. Yet this outcome is not inevitable. Each prescription written with care, each policy limiting unnecessary livestock use, and each investment in prevention shifts the numbers in the right direction. The trajectory of antibiotic resistance is still ours to change if we act now.



